Wed, Feb 4, 2026
Volume 10 - Continuous Publishing
Iran J Neurosurg 2024, 10 - Continuous Publishing: 12-20 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Djientcheu V, Nassourou Oumarou H, Thazo P, Djientcheu C, Fezeu F, Doukas A, et al . Report of the First Completed On-site Vascular Neurosurgery Fellowship Program in Cameroon. Iran J Neurosurg 2024; 10 : 2
URL: http://irjns.org/article-1-385-en.html
URL: http://irjns.org/article-1-385-en.html
Vincent Djientcheu1 

 , Haman Nassourou Oumarou1
, Haman Nassourou Oumarou1 

 , Potho Thazo1
, Potho Thazo1 

 , Carole Djientcheu1
, Carole Djientcheu1 

 , Francis Fezeu2
, Francis Fezeu2 

 , Alexandros Doukas3
, Alexandros Doukas3 

 , Leonidas Trakolis *4
, Leonidas Trakolis *4 

 , Athanasios K. Petridis5
, Athanasios K. Petridis5 




 , Haman Nassourou Oumarou1
, Haman Nassourou Oumarou1 

 , Potho Thazo1
, Potho Thazo1 

 , Carole Djientcheu1
, Carole Djientcheu1 

 , Francis Fezeu2
, Francis Fezeu2 

 , Alexandros Doukas3
, Alexandros Doukas3 

 , Leonidas Trakolis *4
, Leonidas Trakolis *4 

 , Athanasios K. Petridis5
, Athanasios K. Petridis5 


1- Department of Neurosurgery, Faculty of Medicine and Biomedical Sciences, Yaounde General Hospital, University of Yaounde, Yaounde, Cameroon.
2- Department of Neurosurgery, Medical School, Heinrich Heine University Duesseldorf, Düsseldorf, Germany.
3- Department of Neurology and Neurosurgery, Brain Global Non-profit Organization, Salisbury, United States.
4- Department of Neurosurgery, Christian Albrechts University Kiel, Kiel, Germany. ,leonidastra86@hotmail.com
5- Department of Neurosurgery, Faculty of Medicine and Biomedical Sciences, Yaounde General Hospital, University of Yaounde, Yaounde, Cameroon. & Department of Neurology and Neurosurgery, Brain Global Non-profit Organization, Salisbury, United States. & Department of Neurosurgery, Christian Albrechts University Kiel, Kiel, Germany. & Department of Neurosurgery, St. Luke´s Hospital, Thessaloniki, Greece.
2- Department of Neurosurgery, Medical School, Heinrich Heine University Duesseldorf, Düsseldorf, Germany.
3- Department of Neurology and Neurosurgery, Brain Global Non-profit Organization, Salisbury, United States.
4- Department of Neurosurgery, Christian Albrechts University Kiel, Kiel, Germany. ,
5- Department of Neurosurgery, Faculty of Medicine and Biomedical Sciences, Yaounde General Hospital, University of Yaounde, Yaounde, Cameroon. & Department of Neurology and Neurosurgery, Brain Global Non-profit Organization, Salisbury, United States. & Department of Neurosurgery, Christian Albrechts University Kiel, Kiel, Germany. & Department of Neurosurgery, St. Luke´s Hospital, Thessaloniki, Greece.
Keywords: Vascular neurosurgery
fellowship, Sub-saharan
Africa, Brain aneurysm, Micro
neurosurgical clipping
Full Text [PDF 1040 kb]
(1180 Downloads)
| Abstract (HTML) (5919 Views)
References
Full Text: (1144 Views)
1. Introduction
African countries face a shortage of neurosurgeons and neurosurgical units, resulting in increased deaths from acute subarachnoidal hemorrhage (aSAH) [1, 2]. Over the 1.5 years of the fellowship, 38 patients were diagnosed with aSAH in Cameroon. The expected worldwide number of aSAH during this time is 2-22 cases per 100,000 patients per year. For Yaoundé with a population of 2.7 million, the expected aSAH incidence should be 55-608 patients per year [3]. Even with a 25% mortality rate for immediate deaths after aSAH, the incidence should be 41-456 cases per year. However, the incidence rate in Yaoundé is 25 cases per year. Other studies have also reported low aSAH and tumor rates in Africa [4]. Apart from the possibility that aSAH rates in Africa could be caused by fewer patients with brain aneurysms, socioeconomic and cultural factors are more possible reasons for the low aSAH incidence. A higher per capita gross domestic product (GDP), as well as a higher population-to- neurosurgeon ratio was associated with reduced mortality in aSAH [5]. Both these factors are lacking in Africa. Long transportation times to a neurosurgical department have also been shown to increase mortality [6, 7].
To address the higher mortality rates in Cameroon, vascular neurosurgical expertise needs to be established. Therefore, we initiated the on-site fellowship, collaborating with an expert from a high-income country but utilizing the available local equipment.
In the present study, we reported the results of the first on-site cerebrovascular fellowship program, allowing an optimistic view of the future treatment of aneurysms in Cameroon.
2. Materials and Methods
Thirty-eight aneurysm patients, who suffered an aSAH were included and operated during the program. The aim of this first visitation was theoretical teaching and assessment of the available equipment to recognize the level of knowledge and experience regarding neurosurgery and vascular neurosurgery as well as find out what kind of microsurgical equipment was available and what additional needs had to be covered. After this initial assessment, the fellowship was organized as follows:
All the surgeries during the training were done in the presence of the vascular expert who visited the country at specific time points. The trainees were supervised by the expert during their stays, which lasted at least two weeks each time.
The initial surgeries were conducted without the use of a head-holder due to its unavailability. Before the second visit, the vascular neurosurgeon arranged for basic equipment, including a mayfield head holder, various aneurysm clips, micro-dissectors, micro-scissors, and a range of bipolar coagulation forceps. One-third of the cases were solely performed by the trainer, one-third involved both the trainer and trainee and the remaining one-third were carried out by the trainee under the supervision of the trainer. In practice, this approach was implemented as follows:
In the first 10 cases, the trainees familiarized themselves with cerebrovascular neurosurgery approaches, including the supraorbital and pterional approaches. In the subsequent 15 cases, the trainees completed the approach to the aneurysm, while dissection and clipping were performed by the trainer. In the final 13 cases, the trainees independently performed aneurysm clipping under the supervision of the expert. Following this training, the fellows gained sufficient confidence to undertake upcoming cases independently, with the trainer available online for case discussions and treatment strategies. Additionally, two local neurosurgeons (Haman Nassourou Oumarou, Vincent Djientcheu initials blinded-for-review) were trained during this period to assist the trainer in all cases. These fellows were experienced general neurosurgeons, and the department director also had prior experience with vascular neurosurgical cases.
There were four site visits to operate the cases. Online discussions took place on demand when vascular cases came to the hospital and the decision was made if the patients had to be treated during the next site visit. The timetable was as follows:
First site visit (one week): Visiting the hospital and performing three aneurysm surgeries by the expert (Athanasios K. Petridis). In the afternoon, the theoretical part was followed by lectures about surgical approaches and clipping strategies through the visiting expert.
Second site visit (two weeks): Approximately one aneurysm case per day was performed. Postoperative visits and bedside education in the intensive care unit (ICU) and ward management of patients were followed up to share postoperative treatment experiences due to the lack of neuro-anesthesiologists or specialists in neurological care.
Third and fourth site visits (two weeks each): The trainees performed the surgeries under the expert’s supervision; the expert acted only when the trainee could not proceed.
Fifth (last) visit (one week): Microvascular surgery training, including anastomosis, was done in order to acquire better microscopic skills and because of the possibility of aneurysm trapping and IC-EC bypass as most aneurysms that may grow to a large size due to undiagnosis. The bypass operations are in fact technically demanding but do not necessarily require expensive and special technical equipment, both of which would not be available in the country.
During the fellowship program, on-site and online presentations on vascular approaches, clipping strategies, and vascular pathologies, were performed. These presentations included the following 60-minute lessons: 1) Development of neurosurgery, 2) Pathophysiology and diagnosis of SAH, 3) Management of SAH, 4) Supraorbital approach, 5) Pterional approach, 6) Other approaches, 7) Clippology, 8) Microsurgical instruments, 9) Bypasses in vascular neurosurgery, 10) Management of arteriovenous malformation (AVMs), 11) Management of arteriovenous fistulas. 12) Time is life: Delays in diagnosis and transport of patients with SAH to a neurosurgical center. 13) Imaging in vascular neurosurgery (analyzing computed tomographic angiography [CTAs]). Then, an animal training session for anastomoses was organized at the end of the fellowship to establish a training facility (Figure 1).
African countries face a shortage of neurosurgeons and neurosurgical units, resulting in increased deaths from acute subarachnoidal hemorrhage (aSAH) [1, 2]. Over the 1.5 years of the fellowship, 38 patients were diagnosed with aSAH in Cameroon. The expected worldwide number of aSAH during this time is 2-22 cases per 100,000 patients per year. For Yaoundé with a population of 2.7 million, the expected aSAH incidence should be 55-608 patients per year [3]. Even with a 25% mortality rate for immediate deaths after aSAH, the incidence should be 41-456 cases per year. However, the incidence rate in Yaoundé is 25 cases per year. Other studies have also reported low aSAH and tumor rates in Africa [4]. Apart from the possibility that aSAH rates in Africa could be caused by fewer patients with brain aneurysms, socioeconomic and cultural factors are more possible reasons for the low aSAH incidence. A higher per capita gross domestic product (GDP), as well as a higher population-to- neurosurgeon ratio was associated with reduced mortality in aSAH [5]. Both these factors are lacking in Africa. Long transportation times to a neurosurgical department have also been shown to increase mortality [6, 7].
To address the higher mortality rates in Cameroon, vascular neurosurgical expertise needs to be established. Therefore, we initiated the on-site fellowship, collaborating with an expert from a high-income country but utilizing the available local equipment.
In the present study, we reported the results of the first on-site cerebrovascular fellowship program, allowing an optimistic view of the future treatment of aneurysms in Cameroon.
2. Materials and Methods
Thirty-eight aneurysm patients, who suffered an aSAH were included and operated during the program. The aim of this first visitation was theoretical teaching and assessment of the available equipment to recognize the level of knowledge and experience regarding neurosurgery and vascular neurosurgery as well as find out what kind of microsurgical equipment was available and what additional needs had to be covered. After this initial assessment, the fellowship was organized as follows:
All the surgeries during the training were done in the presence of the vascular expert who visited the country at specific time points. The trainees were supervised by the expert during their stays, which lasted at least two weeks each time.
The initial surgeries were conducted without the use of a head-holder due to its unavailability. Before the second visit, the vascular neurosurgeon arranged for basic equipment, including a mayfield head holder, various aneurysm clips, micro-dissectors, micro-scissors, and a range of bipolar coagulation forceps. One-third of the cases were solely performed by the trainer, one-third involved both the trainer and trainee and the remaining one-third were carried out by the trainee under the supervision of the trainer. In practice, this approach was implemented as follows:
In the first 10 cases, the trainees familiarized themselves with cerebrovascular neurosurgery approaches, including the supraorbital and pterional approaches. In the subsequent 15 cases, the trainees completed the approach to the aneurysm, while dissection and clipping were performed by the trainer. In the final 13 cases, the trainees independently performed aneurysm clipping under the supervision of the expert. Following this training, the fellows gained sufficient confidence to undertake upcoming cases independently, with the trainer available online for case discussions and treatment strategies. Additionally, two local neurosurgeons (Haman Nassourou Oumarou, Vincent Djientcheu initials blinded-for-review) were trained during this period to assist the trainer in all cases. These fellows were experienced general neurosurgeons, and the department director also had prior experience with vascular neurosurgical cases.
There were four site visits to operate the cases. Online discussions took place on demand when vascular cases came to the hospital and the decision was made if the patients had to be treated during the next site visit. The timetable was as follows:
First site visit (one week): Visiting the hospital and performing three aneurysm surgeries by the expert (Athanasios K. Petridis). In the afternoon, the theoretical part was followed by lectures about surgical approaches and clipping strategies through the visiting expert.
Second site visit (two weeks): Approximately one aneurysm case per day was performed. Postoperative visits and bedside education in the intensive care unit (ICU) and ward management of patients were followed up to share postoperative treatment experiences due to the lack of neuro-anesthesiologists or specialists in neurological care.
Third and fourth site visits (two weeks each): The trainees performed the surgeries under the expert’s supervision; the expert acted only when the trainee could not proceed.
Fifth (last) visit (one week): Microvascular surgery training, including anastomosis, was done in order to acquire better microscopic skills and because of the possibility of aneurysm trapping and IC-EC bypass as most aneurysms that may grow to a large size due to undiagnosis. The bypass operations are in fact technically demanding but do not necessarily require expensive and special technical equipment, both of which would not be available in the country.
During the fellowship program, on-site and online presentations on vascular approaches, clipping strategies, and vascular pathologies, were performed. These presentations included the following 60-minute lessons: 1) Development of neurosurgery, 2) Pathophysiology and diagnosis of SAH, 3) Management of SAH, 4) Supraorbital approach, 5) Pterional approach, 6) Other approaches, 7) Clippology, 8) Microsurgical instruments, 9) Bypasses in vascular neurosurgery, 10) Management of arteriovenous malformation (AVMs), 11) Management of arteriovenous fistulas. 12) Time is life: Delays in diagnosis and transport of patients with SAH to a neurosurgical center. 13) Imaging in vascular neurosurgery (analyzing computed tomographic angiography [CTAs]). Then, an animal training session for anastomoses was organized at the end of the fellowship to establish a training facility (Figure 1).
Television interviews on national channels and in the newspapers about the program and the vascular pathologies of the central nervous system (CNS) were held to inform the population about the availability of a center. Other health professionals were also informed that there is a reference center where patients with brain aneurysms can be referred to. The patients consented to their surgeries. An IRB/ethics committee approval was not applicable, although the hospital board of directors invited an official expert to develop the program. The participants and any identifiable individuals consented to the publication of their images.
3. Results
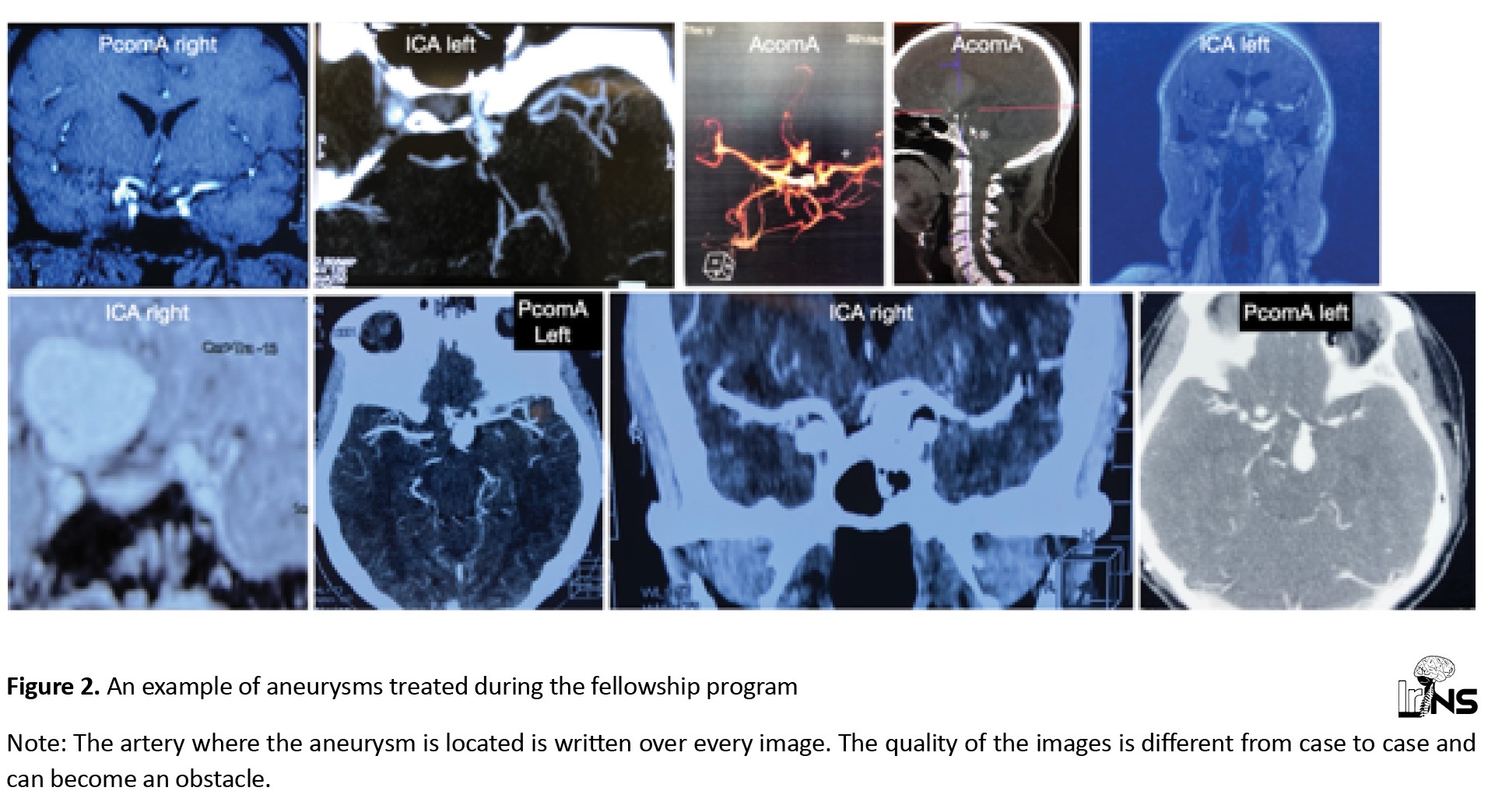
The vascular neurosurgery trainer (AKP) traveled to Yaoundé four times over 1.5 years (April 2021-November 2022) and performed surgeries on 38 brain aneurysm cases, in addition to two AVMs, one Cavernoma, and one spinal arteriovenous fistula (AVF). The aneurysm patients were aSAH survivors, diagnosed with an aneurysm on CTA or MRA (Figure 2).
3. Results
The vascular neurosurgery trainer (AKP) traveled to Yaoundé four times over 1.5 years (April 2021-November 2022) and performed surgeries on 38 brain aneurysm cases, in addition to two AVMs, one Cavernoma, and one spinal arteriovenous fistula (AVF). The aneurysm patients were aSAH survivors, diagnosed with an aneurysm on CTA or MRA (Figure 2).
They were enlisted and planned for surgery during the trainer’s subsequent visit. The mean time from bleeding to surgery was three months. None of the enlisted patients experienced re-bleeding during the waiting period. The indications for surgery were established during online case presentations with the expert.
Demographics
The mean age of the patients was 50 years (ranging from 31 to 66 years). The female-to-male ratio was 1.3:1. The mean size of the aneurysms was 1.5 cm. Aneurysms were distributed as follows: 30.7% in the internal cerebral artery (ICA), 30.7% in the anterior communicating artery (AcomA), 26.9% in the PcomA (posterior communicating artery (PComA), 3.8% in the para ophthalmic, and 7.6% in the medial cerebral artery (MCA).
Thirty-day survival
The 30-day survival rate was 37 out of 38 patients (97.3%). The patient who did not survive was a 42-year-old female with a giant ICA aneurysm measuring 3 cm, who had suffered an aSAH 2 months prior (Figure 3).
Demographics
The mean age of the patients was 50 years (ranging from 31 to 66 years). The female-to-male ratio was 1.3:1. The mean size of the aneurysms was 1.5 cm. Aneurysms were distributed as follows: 30.7% in the internal cerebral artery (ICA), 30.7% in the anterior communicating artery (AcomA), 26.9% in the PcomA (posterior communicating artery (PComA), 3.8% in the para ophthalmic, and 7.6% in the medial cerebral artery (MCA).
Thirty-day survival
The 30-day survival rate was 37 out of 38 patients (97.3%). The patient who did not survive was a 42-year-old female with a giant ICA aneurysm measuring 3 cm, who had suffered an aSAH 2 months prior (Figure 3).
The aneurysm was clipped, and she was discharged on the 7th day post-surgery. Three days after discharge, she experienced generalized convulsions and was admitted to a local hospital. Unfortunately, it was discovered that her intubation narcosis (ITN) tube was dislocated (esophagus), and after 12 hours, she was transferred to our hospital with nonreactive pupils.
Outcome
The intraoperative mortality was 0/38 (0%). All the aneurysms were clipped sufficiently (100%) and opened intraoperatively to ensure occlusion (indocyanine green (ICG) and micro-Doppler were not available). The postoperative CTA of all patients showed perfused adjacent vessels and occluded aneurysms. The postsurgical morbidity was 4/38 (10.5%). Two of these (2/38, 5.2%) patients had permanent morbidity and two cases had temporary neurological symptoms with excellent recovery. The operated patients were survivors of an aSAH with no neurological symptoms on admission. The patient with a significant post-surgical morbidity with long-time ITN and hemiparesis was a 54-year-old female with a ruptured MCA aneurysm and a parietooccipital AVM. The aneurysm was clipped first and the AVM was removed afterward, but with the lack of digital subtraction angiography (DSA), physiologic arteries were occluded causing significant edema. The AVM was Spetzler-Martin grade 2. The second patient, who experienced significant morbidity, had a clipped ICA aneurysm and developed a postoperative hygroma, leading to hemiparesis five days after surgery. The hygroma was drained, and she showed gradual improvement. The third patient was a 62-year-old female with a ruptured right PcomA aneurysm measuring 2.5 cm, along with a right ICA choroidal artery aneurysm measuring 0.7 cm on the same side. During surgery, the PcomA aneurysm ruptured intraoperatively, and temporary clipping of the ICA was performed twice for five minutes each time until the aneurysm was successfully clipped with fenestrated clips, given its location on the posterior ICA wall. Postoperatively, the patient exhibited a hemiparesis of 2/5, and a CCT revealed internal capsule infarction. The fourth patient, a 60-year-old female, presented with a 1 cm AcomA aneurysm and developed asymptomatic neurological Heubner artery infarction.
Two patients with cavernous segment ICA aneurysms experienced oculomotor palsies before surgery, which, as expected, did not immediately improve after intracranial ICA occlusion. Long-term follow-up will reveal further development, although postoperative CTA shows complete ICA and aneurysm thrombosis on the occluded side, with robust hemispheric perfusion from the contralateral side.
We encountered 2 cases of intraoperative rupture, involving PcomA and AcomA aneurysms, which were promptly controlled after a 2-minute proximal temporary occlusion and clipping. The mean operation time from skin to skin was 125 minutes (ranging from 60 to 220 minutes), with craniotomy primarily performed using the gigli saw. Microscope time ranged from 12 to 80 minutes.
Considering that patients with aSAH are treated usually in the first 24 hours after aneurysm rupture in Western countries, there were new considerations in our patient cohort. The microsurgical clipping of intracranial aneurysms ruptured three months ago needs special attention since the basal cisterns can be scarified and the arachnoid surrounding the basal cisterns and the aneurysms is significantly thicker. A major part of dissection cannot be blunt, but has to be sharp Excessive traction on arachnoid tissues to dissect the aneurysm neck should be avoided to prevent tear and re-rupture. Achieving a clear plane of the aneurysm neck is crucial to safely placing a clip around it under visual control of nearby arteries.
In all cases, we employed an external ventricular drainage (EVD) intraoperatively to ensure brain relaxation, facilitating the dissection of the aneurysm as mentioned earlier. The placement of an EVD, according to our experience, is highly recommended and preferred over a lumbar drain, which can also be used, since it allows a more constant and even flow of cerebrospinal fluid and can immediately achieve a reduction of high intracranial pressure in cases of intraoperative rupture of the aneurysm. In two cases, we performed awake ICA occlusion for large and giant symptomatic extradural cavernous segment aneurysms. Since neurophysiology is unavailable, awake ICA-occlusion was the only option to treat these aneurysms. There were another three cases where an aSAH was suspected according to the amount and distribution of blood, but no aneurysm could be seen in the CTA (DSA is not available). In these three cases, exploratory surgery was performed on the side where the blood was dominant, with dissection of the ICA, PcomA, MCA, anterior cerebral artery, A1 segment (A1), AcomA, basilar artery, P1 segment of the posterior cerebral artery, and the proximal part of the superior cerebellar artery (SCA; ipsilateral and contralateral). In two cases, we did not find a bleeding source. However, in one case, we could identify a 2 mm ICA-ophthalmic artery aneurysm, which was clipped.
Other neurovascular pathologies
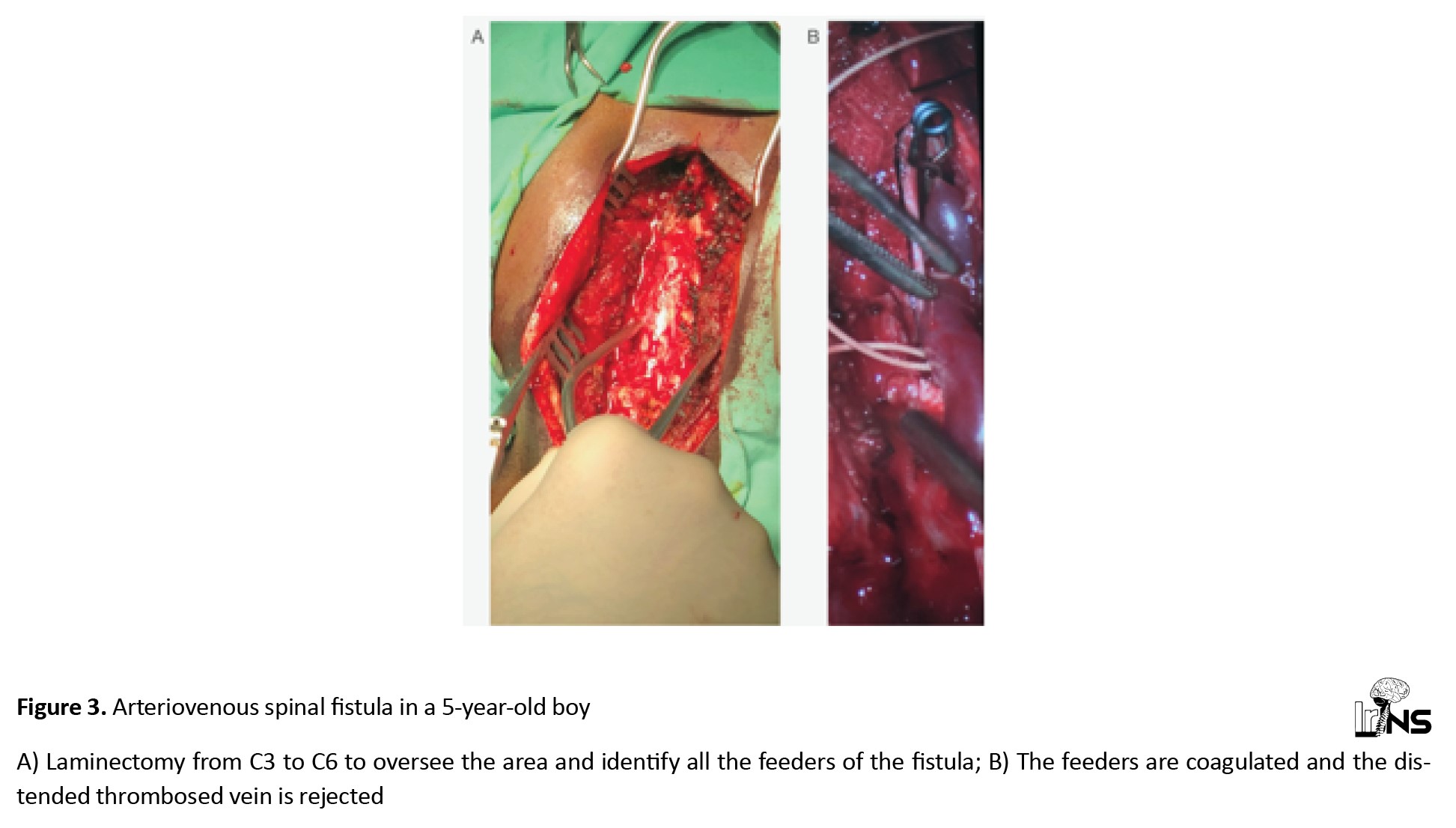
In addition to aneurysm surgery, a hemorrhaged cerebellar cavernoma in a 42-year-old female was removed. Another case involved a 5-year-old boy admitted to the neurosurgery department with a dural cervical spine AVF spanning levels cervical vertebra 3 (C3)-cervical vertebra 6 (C6), which caused paraparesis (grade 2/5) and rendered the patient bedridden for the past two months. A multilevel laminectomy of the cervical spine was performed to identify and occlude the fistula. The thrombosed vein, compressing the spinal cord, was removed, as well. Six months after surgery, he was able to walk without assistance (Figure 3).
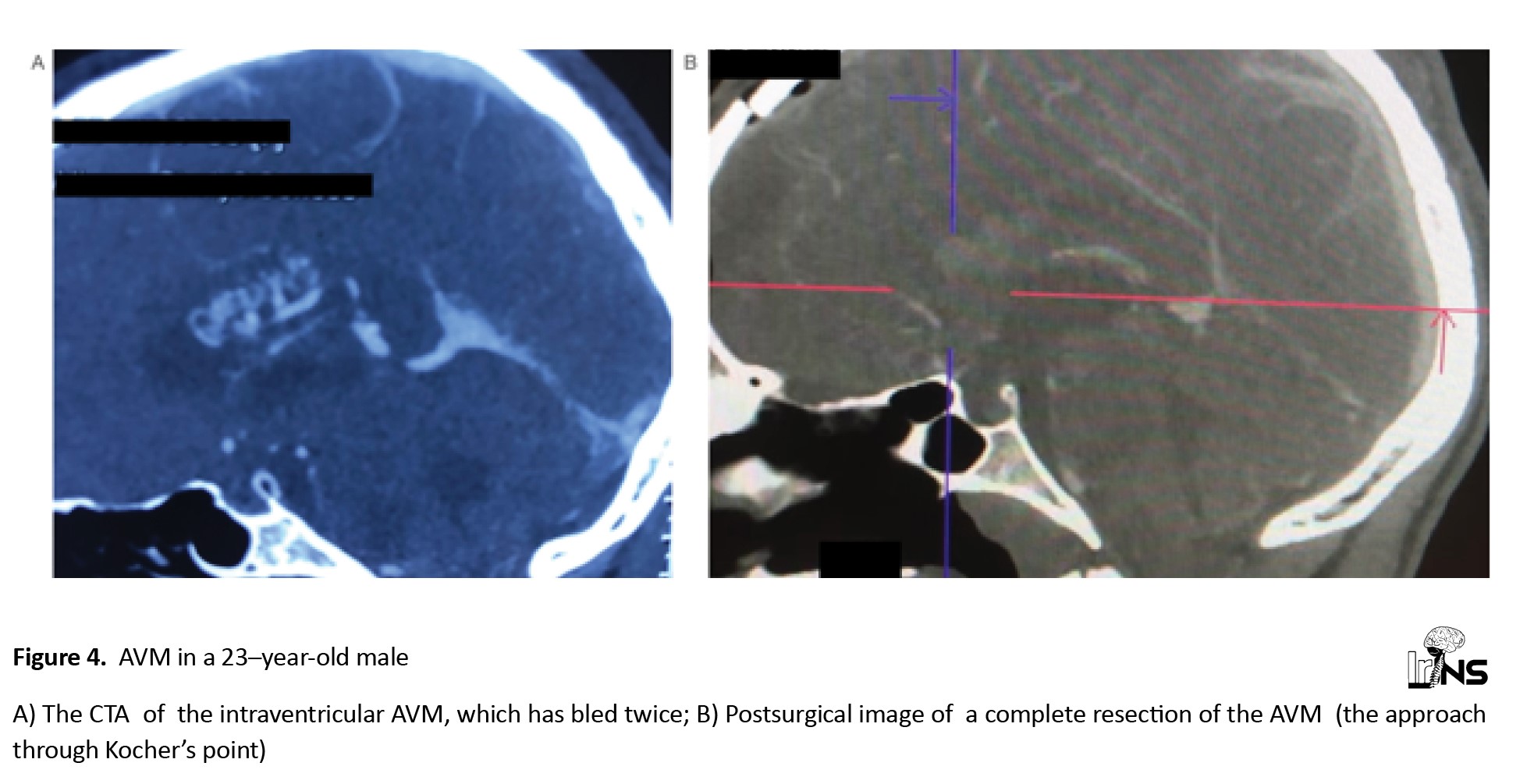
The last case of a non-aneurysmatic pathology was a 23-year-old male with two times intraventricular AVM bleeding. The lateral ventricle was opened through Kocher’s point and the AVM was completely removed (Figure 4).
Outcome
The intraoperative mortality was 0/38 (0%). All the aneurysms were clipped sufficiently (100%) and opened intraoperatively to ensure occlusion (indocyanine green (ICG) and micro-Doppler were not available). The postoperative CTA of all patients showed perfused adjacent vessels and occluded aneurysms. The postsurgical morbidity was 4/38 (10.5%). Two of these (2/38, 5.2%) patients had permanent morbidity and two cases had temporary neurological symptoms with excellent recovery. The operated patients were survivors of an aSAH with no neurological symptoms on admission. The patient with a significant post-surgical morbidity with long-time ITN and hemiparesis was a 54-year-old female with a ruptured MCA aneurysm and a parietooccipital AVM. The aneurysm was clipped first and the AVM was removed afterward, but with the lack of digital subtraction angiography (DSA), physiologic arteries were occluded causing significant edema. The AVM was Spetzler-Martin grade 2. The second patient, who experienced significant morbidity, had a clipped ICA aneurysm and developed a postoperative hygroma, leading to hemiparesis five days after surgery. The hygroma was drained, and she showed gradual improvement. The third patient was a 62-year-old female with a ruptured right PcomA aneurysm measuring 2.5 cm, along with a right ICA choroidal artery aneurysm measuring 0.7 cm on the same side. During surgery, the PcomA aneurysm ruptured intraoperatively, and temporary clipping of the ICA was performed twice for five minutes each time until the aneurysm was successfully clipped with fenestrated clips, given its location on the posterior ICA wall. Postoperatively, the patient exhibited a hemiparesis of 2/5, and a CCT revealed internal capsule infarction. The fourth patient, a 60-year-old female, presented with a 1 cm AcomA aneurysm and developed asymptomatic neurological Heubner artery infarction.
Two patients with cavernous segment ICA aneurysms experienced oculomotor palsies before surgery, which, as expected, did not immediately improve after intracranial ICA occlusion. Long-term follow-up will reveal further development, although postoperative CTA shows complete ICA and aneurysm thrombosis on the occluded side, with robust hemispheric perfusion from the contralateral side.
We encountered 2 cases of intraoperative rupture, involving PcomA and AcomA aneurysms, which were promptly controlled after a 2-minute proximal temporary occlusion and clipping. The mean operation time from skin to skin was 125 minutes (ranging from 60 to 220 minutes), with craniotomy primarily performed using the gigli saw. Microscope time ranged from 12 to 80 minutes.
Considering that patients with aSAH are treated usually in the first 24 hours after aneurysm rupture in Western countries, there were new considerations in our patient cohort. The microsurgical clipping of intracranial aneurysms ruptured three months ago needs special attention since the basal cisterns can be scarified and the arachnoid surrounding the basal cisterns and the aneurysms is significantly thicker. A major part of dissection cannot be blunt, but has to be sharp Excessive traction on arachnoid tissues to dissect the aneurysm neck should be avoided to prevent tear and re-rupture. Achieving a clear plane of the aneurysm neck is crucial to safely placing a clip around it under visual control of nearby arteries.
In all cases, we employed an external ventricular drainage (EVD) intraoperatively to ensure brain relaxation, facilitating the dissection of the aneurysm as mentioned earlier. The placement of an EVD, according to our experience, is highly recommended and preferred over a lumbar drain, which can also be used, since it allows a more constant and even flow of cerebrospinal fluid and can immediately achieve a reduction of high intracranial pressure in cases of intraoperative rupture of the aneurysm. In two cases, we performed awake ICA occlusion for large and giant symptomatic extradural cavernous segment aneurysms. Since neurophysiology is unavailable, awake ICA-occlusion was the only option to treat these aneurysms. There were another three cases where an aSAH was suspected according to the amount and distribution of blood, but no aneurysm could be seen in the CTA (DSA is not available). In these three cases, exploratory surgery was performed on the side where the blood was dominant, with dissection of the ICA, PcomA, MCA, anterior cerebral artery, A1 segment (A1), AcomA, basilar artery, P1 segment of the posterior cerebral artery, and the proximal part of the superior cerebellar artery (SCA; ipsilateral and contralateral). In two cases, we did not find a bleeding source. However, in one case, we could identify a 2 mm ICA-ophthalmic artery aneurysm, which was clipped.
Other neurovascular pathologies
In addition to aneurysm surgery, a hemorrhaged cerebellar cavernoma in a 42-year-old female was removed. Another case involved a 5-year-old boy admitted to the neurosurgery department with a dural cervical spine AVF spanning levels cervical vertebra 3 (C3)-cervical vertebra 6 (C6), which caused paraparesis (grade 2/5) and rendered the patient bedridden for the past two months. A multilevel laminectomy of the cervical spine was performed to identify and occlude the fistula. The thrombosed vein, compressing the spinal cord, was removed, as well. Six months after surgery, he was able to walk without assistance (Figure 3).
The last case of a non-aneurysmatic pathology was a 23-year-old male with two times intraventricular AVM bleeding. The lateral ventricle was opened through Kocher’s point and the AVM was completely removed (Figure 4).
The patient was discharged without neurological disturbances eight days post-surgery.
Long-term follow-up
Six patients underwent a 12-month follow-up. One patient reported visual acuity issues. Among them, 4 out of 6 (67.9%) had a modified rankin score of 0, indicating no disability, while 2 out of 6 (33.3%) had a modified Rankin score of 1, signifying minor disability.
Post-fellowship evaluation of cases operated by the fellows without any supervision
After completion of the fellowship program, the new vascular neurosurgeons were able to compare their results before and after the fellowship. Before the fellowship, 13 patients were operated for aneurysms without SAH with an average operating time of 5 hours and 30 minutes, resulting in 2 deaths within the first 30 days and 2 significant post-surgical morbidities. After completing the vascular fellowship, the two local trainees performed surgeries on 15 cases of non-ruptured aneurysms without any surgical complications or deaths. The operating time was reduced to 4 hours and 15 minutes. The first reference vascular neurosurgical center in Cameroon has been established now. Equipped with diagnostic imaging capabilities, like CTA, and staffed with trained vascular neurosurgeons, the center can independently manage CNS vascular pathologies. The center will provide care for aSAH patients across the entire country and will also train local neurosurgeons in order to establish additional centers in Cameroon. The long-term plan is to proceed with the same strategy in a number of Sub-Saharan countries.
4. Discussion
Mainly Morocco, Egypt, and South Africa have reported on aSAH [1]. Senegal shared their experience with 102 ruptured intracranial aneurysms over approximately 3 years [8]. Cameroon has not reported on its aneurysm experience until now, mainly due to the lack of well-established caseloads and management. The main treatment strategy for cerebral aneurysms in Africa is microsurgery and much less endovascular treatment [1]. A study reported about 792 patients treated micro-surgically vs. 94 endovascularly, and since countries, like Morocco, South Africa, and Egypt are included, the number of endovascular procedures is overestimated [1]. Therefore, training has to be concentrated on microsurgical expertise, which is economically feasible. We agree with Tetinou et al. on the reasons for the low incidence of aSAH, attributed to the lack of neuroimaging and neurosurgical facilities [1]. A favorable outcome was reported in 26.2% of cases, with a mortality rate of 7.9% [1]. In comparison, mortality rates from the USA for unruptured aneurysms range from 1.4% to 3.5% [9, 10] and for ruptured aneurysms, it is 8.9% [11]. Our results show better outcomes, indicating that the quality of surgery depends mostly on the expertise of the surgeons rather than the equipment. The establishment of quality cerebral aneurysm treatment in Africa will rely on well-trained experts. Our patients experienced a ruptured aneurysm a few months before surgery, survived the aSAH, and were operated on later; therefore, they cannot be counted as ruptured nor as un-ruptured aneurysms. Surgery on these aneurysms is not as straightforward as on unruptured ones because scar tissue and hypertrophied arachnoid villi around the aneurysm necessitate sharp dissection. Nonetheless, we experienced no intraoperative mortality, a 30-day mortality rate of 2.7%, and a functional morbidity rate of 10.5%, with a favorable outcome in 89% of patients, closely resembling the post-clipping outcomes for unruptured aneurysms in the Western world. The one-year follow-up on our patients showed an mRS of zero in 67% and one in 33%, indicating that the post-surgical recovery at least for a number of patients was excellent. The size of the treated aneurysm was bigger than the ones we treat in our Western population since diagnostics are often delayed in Africa. The success of the program is evident in the significant improvement in the surgical times of local fellows and an intraoperative mortality and complication rate of 0% in the surgical treatment of unruptured intracranial aneurysms.
There is a report from Senegal indicating a high rate of pediatric intracranial aneurysm in the African population [12]. Fortunately, we did not have such a high incidence. The only child we treated for vascular pathology was a 5-year-old boy with a spinal AVF. Spinal DSA was not available, but based on the MRI findings, we suspected a Spetzler type IV fistula [13]. Therefore, we anticipated multipediculated feeders, a giant fistula, and dilated tortuous veins, and proceeded with a multilevel laminectomy to access multiple levels and both sides. As reported in another case report of a 12-year-old female, she needed nine months after endovascular treatment of a conus medullaris AVF to gain complete recovery [14]. Our patient was able to walk after six months, although his gait remains unstable. We expect further improvement in the one-year follow-up.
Following the successful completion of the first cerebrovascular fellowship program in Cameroon, other areas in the country and other Central African countries should follow this example. By establishing more training centers within the same country, the time from aSAH diagnosis to treatment could be significantly reduced. The mean time from diagnosis to treatment in Africa is 12.1 days, whereas in Western countries, this time is 26.7 hours [15]. It is well known that a delay from diagnosis to treatment increases the mortality of aSAH patients [6, 7].
Togo has also a significantly low rate of aSAH in its population, which indicates the problems mentioned above (lack of diagnosis and treatment options) [16]. It seems that there is a need to expand our program to countries where the incidence rates are very low, in accordance with a low number of neurosurgical centers.
We expect a higher aSAH incidence after the center is established. One report from Nigeria estimated that 6 million people will develop a brain aneurysm throughout their lifetime and will need treatment. For the Cameroonian population, we expect this number to be 1.28 million. These patients will require centers of excellence to receive accurate management of their aneurysms.
Apart from the microsurgical training in the operating room, the expert has to follow the patient’s treatment in the ICU and also train the anesthesiologist. It has been reported that patients in Africa are twice as likely to die after surgery compared to the global average for postoperative deaths [17].
5. Conclusion
We reported the results of the first completed on-site cerebrovascular fellowship in Cameroon. The surgical outcomes of the cerebrovascular expert in his cases in Africa, were similar to the Western world and the outcomes of the fellows after completion of the program showed a significant improvement. Training local neurosurgeons in specified areas with on-site fellowships will lead to a significant improvement in the treatment quality in cerebrovascular cases. Establishing a continuous mentorship program over a long period of time should be difficult but achievable.
Limitations
The fellowship was based on one expert whose time of stay in Cameroon was limited due to other responsibilities in his own department. The fellows learned only one expert’s technique. The time for the follow-up of the cases performed by the trainees is still limited. More cases and longer follow-ups will demonstrate the experience gained from the trainees. The ideal fellowship training team would consist of two training experts, one neuro-anesthesiologist, and one neuro-ICU expert.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research; although the hospital board of directors invited the expert officially to establish the program.
Funding
This research was financially supported by the Global Brain Organization.
Authors' contributions
Conceptualization and study design, data collection, data analysis, interpretatio: All authors; Critically revising: Alexandros Doukas, Leonidas Trakolis and Athanasios Petridis; Review and editing: Leonidas Trakolis and Athanasios Petridis; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
Long-term follow-up
Six patients underwent a 12-month follow-up. One patient reported visual acuity issues. Among them, 4 out of 6 (67.9%) had a modified rankin score of 0, indicating no disability, while 2 out of 6 (33.3%) had a modified Rankin score of 1, signifying minor disability.
Post-fellowship evaluation of cases operated by the fellows without any supervision
After completion of the fellowship program, the new vascular neurosurgeons were able to compare their results before and after the fellowship. Before the fellowship, 13 patients were operated for aneurysms without SAH with an average operating time of 5 hours and 30 minutes, resulting in 2 deaths within the first 30 days and 2 significant post-surgical morbidities. After completing the vascular fellowship, the two local trainees performed surgeries on 15 cases of non-ruptured aneurysms without any surgical complications or deaths. The operating time was reduced to 4 hours and 15 minutes. The first reference vascular neurosurgical center in Cameroon has been established now. Equipped with diagnostic imaging capabilities, like CTA, and staffed with trained vascular neurosurgeons, the center can independently manage CNS vascular pathologies. The center will provide care for aSAH patients across the entire country and will also train local neurosurgeons in order to establish additional centers in Cameroon. The long-term plan is to proceed with the same strategy in a number of Sub-Saharan countries.
4. Discussion
Mainly Morocco, Egypt, and South Africa have reported on aSAH [1]. Senegal shared their experience with 102 ruptured intracranial aneurysms over approximately 3 years [8]. Cameroon has not reported on its aneurysm experience until now, mainly due to the lack of well-established caseloads and management. The main treatment strategy for cerebral aneurysms in Africa is microsurgery and much less endovascular treatment [1]. A study reported about 792 patients treated micro-surgically vs. 94 endovascularly, and since countries, like Morocco, South Africa, and Egypt are included, the number of endovascular procedures is overestimated [1]. Therefore, training has to be concentrated on microsurgical expertise, which is economically feasible. We agree with Tetinou et al. on the reasons for the low incidence of aSAH, attributed to the lack of neuroimaging and neurosurgical facilities [1]. A favorable outcome was reported in 26.2% of cases, with a mortality rate of 7.9% [1]. In comparison, mortality rates from the USA for unruptured aneurysms range from 1.4% to 3.5% [9, 10] and for ruptured aneurysms, it is 8.9% [11]. Our results show better outcomes, indicating that the quality of surgery depends mostly on the expertise of the surgeons rather than the equipment. The establishment of quality cerebral aneurysm treatment in Africa will rely on well-trained experts. Our patients experienced a ruptured aneurysm a few months before surgery, survived the aSAH, and were operated on later; therefore, they cannot be counted as ruptured nor as un-ruptured aneurysms. Surgery on these aneurysms is not as straightforward as on unruptured ones because scar tissue and hypertrophied arachnoid villi around the aneurysm necessitate sharp dissection. Nonetheless, we experienced no intraoperative mortality, a 30-day mortality rate of 2.7%, and a functional morbidity rate of 10.5%, with a favorable outcome in 89% of patients, closely resembling the post-clipping outcomes for unruptured aneurysms in the Western world. The one-year follow-up on our patients showed an mRS of zero in 67% and one in 33%, indicating that the post-surgical recovery at least for a number of patients was excellent. The size of the treated aneurysm was bigger than the ones we treat in our Western population since diagnostics are often delayed in Africa. The success of the program is evident in the significant improvement in the surgical times of local fellows and an intraoperative mortality and complication rate of 0% in the surgical treatment of unruptured intracranial aneurysms.
There is a report from Senegal indicating a high rate of pediatric intracranial aneurysm in the African population [12]. Fortunately, we did not have such a high incidence. The only child we treated for vascular pathology was a 5-year-old boy with a spinal AVF. Spinal DSA was not available, but based on the MRI findings, we suspected a Spetzler type IV fistula [13]. Therefore, we anticipated multipediculated feeders, a giant fistula, and dilated tortuous veins, and proceeded with a multilevel laminectomy to access multiple levels and both sides. As reported in another case report of a 12-year-old female, she needed nine months after endovascular treatment of a conus medullaris AVF to gain complete recovery [14]. Our patient was able to walk after six months, although his gait remains unstable. We expect further improvement in the one-year follow-up.
Following the successful completion of the first cerebrovascular fellowship program in Cameroon, other areas in the country and other Central African countries should follow this example. By establishing more training centers within the same country, the time from aSAH diagnosis to treatment could be significantly reduced. The mean time from diagnosis to treatment in Africa is 12.1 days, whereas in Western countries, this time is 26.7 hours [15]. It is well known that a delay from diagnosis to treatment increases the mortality of aSAH patients [6, 7].
Togo has also a significantly low rate of aSAH in its population, which indicates the problems mentioned above (lack of diagnosis and treatment options) [16]. It seems that there is a need to expand our program to countries where the incidence rates are very low, in accordance with a low number of neurosurgical centers.
We expect a higher aSAH incidence after the center is established. One report from Nigeria estimated that 6 million people will develop a brain aneurysm throughout their lifetime and will need treatment. For the Cameroonian population, we expect this number to be 1.28 million. These patients will require centers of excellence to receive accurate management of their aneurysms.
Apart from the microsurgical training in the operating room, the expert has to follow the patient’s treatment in the ICU and also train the anesthesiologist. It has been reported that patients in Africa are twice as likely to die after surgery compared to the global average for postoperative deaths [17].
5. Conclusion
We reported the results of the first completed on-site cerebrovascular fellowship in Cameroon. The surgical outcomes of the cerebrovascular expert in his cases in Africa, were similar to the Western world and the outcomes of the fellows after completion of the program showed a significant improvement. Training local neurosurgeons in specified areas with on-site fellowships will lead to a significant improvement in the treatment quality in cerebrovascular cases. Establishing a continuous mentorship program over a long period of time should be difficult but achievable.
Limitations
The fellowship was based on one expert whose time of stay in Cameroon was limited due to other responsibilities in his own department. The fellows learned only one expert’s technique. The time for the follow-up of the cases performed by the trainees is still limited. More cases and longer follow-ups will demonstrate the experience gained from the trainees. The ideal fellowship training team would consist of two training experts, one neuro-anesthesiologist, and one neuro-ICU expert.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research; although the hospital board of directors invited the expert officially to establish the program.
Funding
This research was financially supported by the Global Brain Organization.
Authors' contributions
Conceptualization and study design, data collection, data analysis, interpretatio: All authors; Critically revising: Alexandros Doukas, Leonidas Trakolis and Athanasios Petridis; Review and editing: Leonidas Trakolis and Athanasios Petridis; Final approval: All authors.
Conflict of interest
The authors declared no conflict of interest.
References
- Tetinou F, Kanmounye US, Sadler S, Nitcheu I, Oriaku AJ, Ndajiwo AB, et al. Cerebral aneurysms in Africa: A scoping review. Interdisciplinary Neurosurgery. 2021; 26:101291. [DOI:10.1016/j.inat.2021.101291]
- Ogungbo B, Mendelow AD, Walker R. The epidemiology, diagnosis and treatment of subarachnoid haemorrhage in Nigeria: What do we know and what do we need to know? British Journal of Neurosurgery. 2004; 18(4):362-6. [DOI:10.1080/02688690400005057] [PMID]
- Petridis AK, Kamp MA, Cornelius JF, Beez T, Beseoglu K, Turowski B, et al. Aneurysmal subarachnoid hemorrhage. Deutsches Arzteblatt International. 2017; 114(13):226-36. [DOI:10.3238/arztebl.2017.0226] [PMID] [PMCID]
- Ohaegbulam SC. Geographical neurosurgery. Neurological Research. 1999; 21(2):161-70. [DOI:10.1080/01616412.1999.11740912] [PMID]
- Guha D, Ibrahim GM, Kertzer JD, Macdonald RL. National socioeconomic indicators are associated with outcomes after aneurysmal subarachnoid hemorrhage: A hierarchical mixed-effects analysis. Journal of Neurosurgery. 2014; 121(5):1039-47. [DOI:10.3171/2014.7.JNS132141] [PMID]
- van Lieshout JH, Bruland I, Fischer I, Cornelius JF, Kamp MA, Turowski B, et al. Increased mortality of patients with aneurysmatic subarachnoid hemorrhage caused by prolonged transport time to a high-volume neurosurgical unit. The American Journal of Emergency Medicine. 2017; 35(1):45-50. [DOI:10.1016/j.ajem.2016.09.067] [PMID]
- Doukas A, Barth H, Petridis KA, Mehdorn M, von der Brelie C. Misdiagnosis of acute subarachnoid hemorrhage in the era of multimodal diagnostic options. he American Journal of Emergency Medicine. 2019; 37(11):2079-83. [DOI:10.1016/j.ajem.2019.03.001] [PMID]
- Thioub M, Mbaye M, Thiam AB, Zirhumana C, Sy C, Ndoye N, et al. Microsurgical treatment of ruptured intracranial aneurysms in Sub-Saharan Africa: A series of 102 consecutive cases treated in senegal. World Neurosurgery. 2018; 110:226-31. [DOI:10.1016/j.wneu.2017.11.048] [PMID]
- Bekelis K, Missios S, MacKenzie TA. Outcomes of elective cerebral aneurysm treatment performed by attending neurosurgeons after night work. Neurosurgery. 2018; 82(3):329-34. [DOI:10.1093/neuros/nyx174] [PMID] [PMCID]
- Johnston SC, Zhao S, Dudley RA, Berman MF, Gress DR. Treatment of unruptured cerebral aneurysms in California. Stroke. 2001; 32(3):597-605. [DOI:10.1161/01.STR.32.3.597] [PMID]
- Fraser JF, Riina H, Mitra N, Gobin YP, Simon AS, Stieg PE. Treatment of ruptured intracranial aneurysms: Looking to the past to register the future. Neurosurgery. 2006; 59(6):1157-66. [DOI:10.1227/01.NEU.0000245623.70344.F7] [PMID]
- Thioub M, Mbaye M, Thiam AB, Mutomb S, Sy C, Faye M, et al. Pediatric intracranial aneurysms in Senegal: A series of 10 cases treated in unfavorable socio-economic conditions. Child's Nervous System. 2019; 35(1):165-8. [DOI:10.1007/s00381-018-3943-2] [PMID]
- Spetzler RF, Detwiler PW, Riina HA, Porter RW. Modified classification of spinal cord vascular lesions. Journal of Neurosurgery. 2002; 96(2 Suppl):145-56. [DOI:10.3171/spi.2002.96.2.0145] [PMID]
- Bankole NDA, Janot K, Lystrat A, Travers N, Maldonado IL, Velut S. Child pial arteriovenous fistula of the conus medullaris presenting with spinal cord venous congestion: Case report and literature review. Interdisciplinary Neurosurgery. 2021; 25:101128. [DOI:10.1016/j.inat.2021.101128]
- Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. Journal of Neurosurgery. 1999; 90(6):1018-24. [DOI:10.3171/jns.1999.90.6.1018] [PMID]
- Ahanogbe KM, Belo M, Beketi AK, Kpelao S, Doleagbenou KA. [Problematics of subarachnoid hemorrhage in developing countries: The case of Togo (French)]. Neuro-Chirurgie. 2016; 62(6):312-6. [DOI:10.1016/j.neuchi.2016.06.008] [PMID]
- Biccard BM, Madiba TE, Kluyts HL, Munlemvo DM, Madzimbamuto FD, Basenero A, et al. Perioperative patient outcomes in the African surgical outcomes study: A 7-day prospective observational cohort study. Lancet. 2018; 391(10130):1589-98. [DOI:10.1016/S0140-6736(18)30001-1] [PMID]
Type of Study: Special Article |
Subject:
Vascular Neurosurgery
Send email to the article author
| Rights and Permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |